Background: The TP53 gene is mutated in approximately half of human cancers and in up to 20% of acute myeloid leukemia (AML). TP53 mutations are associated with complex karyotype, high rates of resistance to standard treatment, and dismal outcome. However, new AML therapies are being developed that may potentially target these mutations (e.g. APR-246) or are largely ineffective in the context of mutated TP53 (e.g. MDM2 inhibitors). We sought to evaluate the frequency of treatment-emergent TP53 mutations in patients (pts) with TP53 wild type (WT) AML who relapsed or were refractory to therapy.
Methods: This is a retrospective analysis of 1293 pts with WT TP53 AML who were treated at our institution with frontline therapy between December 2012 and March 2020. Pts with core binding factor (CBF) AML, those who did not have mutational data at baseline, or those in whom mutation profiling was not performed at relapse were excluded from the analysis. To identify TP53 mutations at baseline or relapse, a targeted next-generation sequencing (NGS) platform was performed. The analytical sensitivity of this NGS panel was established at 1-2% mutant reads in a background of WT reads. Mutations were manually reviewed to exclude any artifacts.
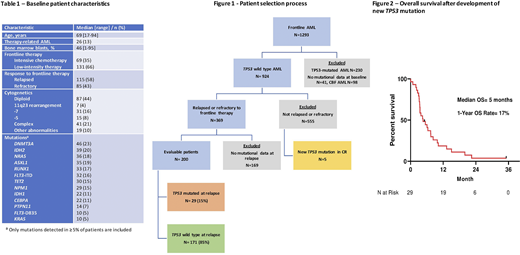
Results: We identified 200 pts with non-CBF TP53 WT AML at diagnosis who relapsed after or were refractory to frontline therapy and are the subject of this analysis. Figure 1 shows the pt selection process. The baseline characteristics are shown in Table 1. At diagnosis, the median age was 69 years (range, 17-94 years). By ELN classification, 85 pts (43%) were adverse risk, 94 (47%) were intermediate risk, and 21 (11%) were favorable risk. Eighty-five pts (43%) were refractory to frontline therapy, and 115 (58%) relapsed after frontline therapy. Sixty-nine pts (35%) received intensive chemotherapy and 131 pts (66%) receiver low-intensity therapy.
Overall, 29 pts (15%) developed a newly detectable TP53 mutation at some point over the course of therapy in the context of relapsed/refractory disease. Nineteen of these pts (66%) acquired a new detectable mutation after the first line of therapy, 6 pts (21%) after two lines of therapy, and 4 pts (14%) after three lines of therapy. Twenty-four pts (83%) acquired 1 TP53 mutation and 5 pts (17%) developed 2 TP53 mutations. The median variant allelic frequency (VAF) of the TP53 mutation was 15% (range 1.1% - 95.6%).
Baseline factors associated with an increased likelihood of developing a new TP53 mutation included chromosome 5 abnormality (new TP53 mutation 40% vs. 12% in those without; P=0.02) and presence of an IDH2 mutation (28% vs. 12%, respectively; P=0.02). Development of a new TP53 mutation was more common after high-intensity therapy (23% vs. 10% after low-intensity therapy; P=0.02) and were also more common after hematopoietic stem cell transplant (HSCT) compared to those who did not undergo HSCT (36% vs. 12%, respectively; P=0.005).
Thirteen (45%) of the new TP53 mutations occurred in the context of complex cytogenetics. Interestingly, in 7 of these cases, both the cytogenetic complexity and TP53 mutation emerged concomitantly and were not present at baseline. Among pts who developed new detectable TP53 mutations, the most common co-mutations were DDX41, DNMT3A, IDH2 and NRAS (each present in 15-20% of TP53-mutated cases).
Seventeen (59%) of the 29 pts with new TP53 mutation responded to salvage therapy, and 7 underwent subsequent HSCT. Overall, survival was poor after development of newly detected TP53 mutation. The median overall survival (OS) after acquisition of TP53 mutation was 5.0 months, with a 1-year OS rate of 17% (Figure 2).
Interestingly, we also identified a new detectable TP53 mutation in 5 pts (1%) out of the 555 pts who were in complete remission. The median VAF at the time of mutant TP53 acquisition was 2.5% (range 1.0% - 3.3%). With a median follow-up of 27 months since detection of the new TP53 mutation, none of these pts have developed hematologic relapse.
Conclusion: We identified one or more newly detectable TP53 mutation(s) over the course of disease in 15% pts with relapsed/refractory AML. These mutations tended to be subclonal with a median VAF of 15%. New TP53 mutations were more common after intensive chemotherapy and/or HSCT. Our data suggest that these mutations may be acquired throughout the course of therapy, and therefore sequential monitoring may be relevant in the era of new potential TP53-targeting therapies.
Daver:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Konopleva:Eli Lilly: Research Funding; Agios: Research Funding; Cellectis: Research Funding; Amgen: Consultancy; AbbVie: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Ablynx: Research Funding; Kisoji: Consultancy; AstraZeneca: Research Funding; Ascentage: Research Funding; Genentech: Consultancy, Research Funding; Calithera: Research Funding; Forty-Seven: Consultancy, Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Sanofi: Research Funding. Ravandi:Celgene: Consultancy, Honoraria; Xencor: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Kadia:Astellas: Research Funding; Abbvie: Honoraria, Research Funding; JAZZ: Honoraria, Research Funding; Amgen: Research Funding; Novartis: Honoraria; Incyte: Research Funding; Astra Zeneca: Research Funding; Pfizer: Honoraria, Research Funding; Cellenkos: Research Funding; Cyclacel: Research Funding; Ascentage: Research Funding; Celgene: Research Funding; BMS: Honoraria, Research Funding; Pulmotec: Research Funding; Genentech: Honoraria, Research Funding. DiNardo:MedImmune: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Syros: Honoraria; Jazz: Honoraria. Issa:Celegene: Research Funding; Syndax: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Borthakur:Oncoceutics: Research Funding; BioLine Rx: Research Funding; Treadwell Therapeutics: Consultancy; Xbiotech USA: Research Funding; BMS: Research Funding; AstraZeneca: Research Funding; PTC Therapeutics: Research Funding; Incyte: Research Funding; Polaris: Research Funding; Jannsen: Research Funding; GSK: Research Funding; Novartis: Research Funding; Abbvie: Research Funding; Curio Science LLC: Consultancy; FTC Therapeutics: Consultancy; Argenx: Consultancy; PTC Therapeutics: Consultancy; BioLine Rx: Consultancy; BioTherix: Consultancy; Nkarta Therapeutics: Consultancy; Cyclacel: Research Funding. Yilmaz:Daicho Sankyo: Research Funding; Pfizer: Research Funding; Pint Pharma: Honoraria. Maiti:Celgene: Research Funding. Kantarjian:Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cyclacel: Research Funding; Daiichi-Sankyo: Research Funding; Astex: Research Funding; Immunogen: Research Funding; Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding; BMS: Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Honoraria; Amgen: Honoraria, Research Funding; Ariad: Research Funding; Agios: Honoraria, Research Funding; Novartis: Research Funding. Short:AstraZeneca: Consultancy; Amgen: Honoraria; Astellas: Research Funding; Takeda Oncology: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal